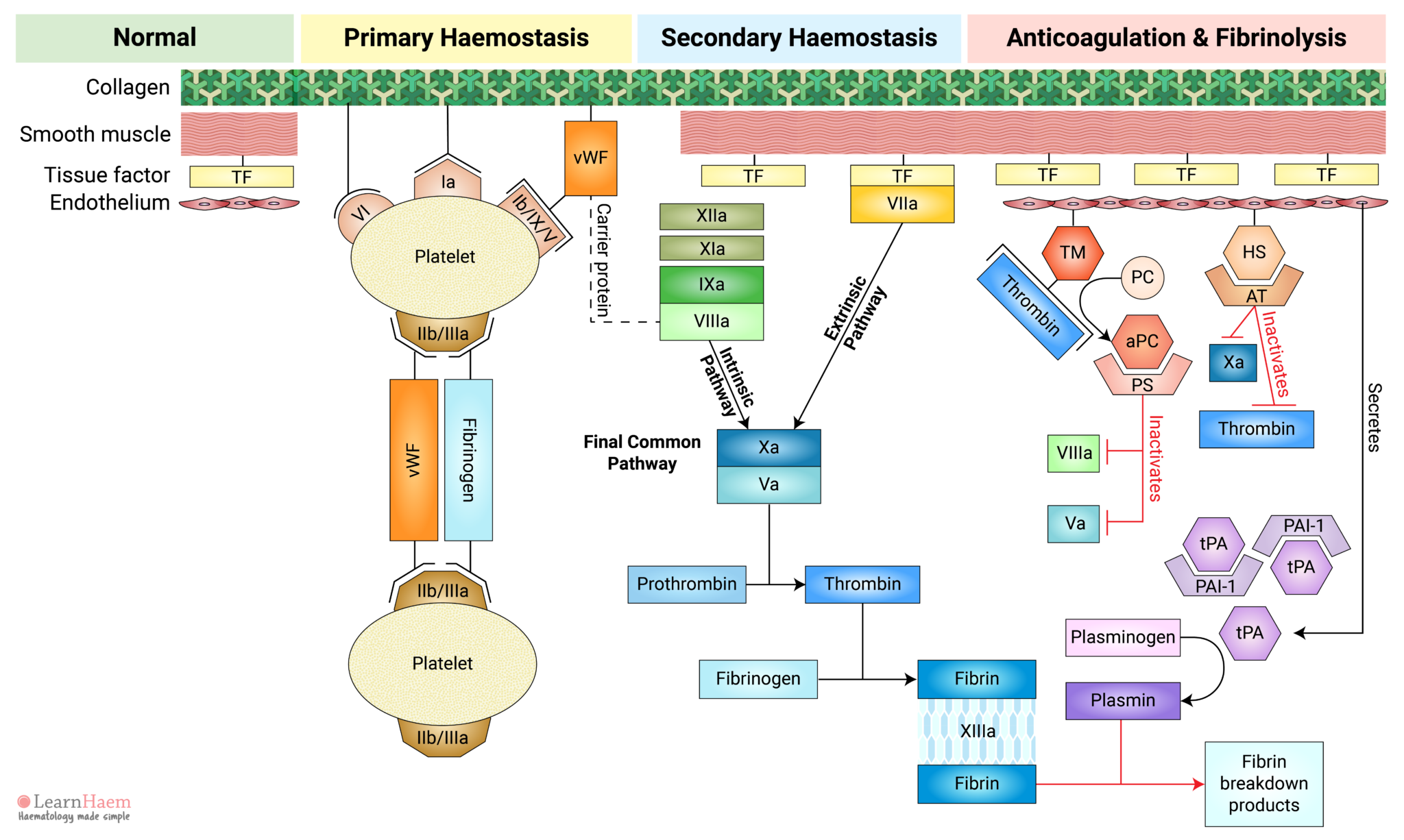
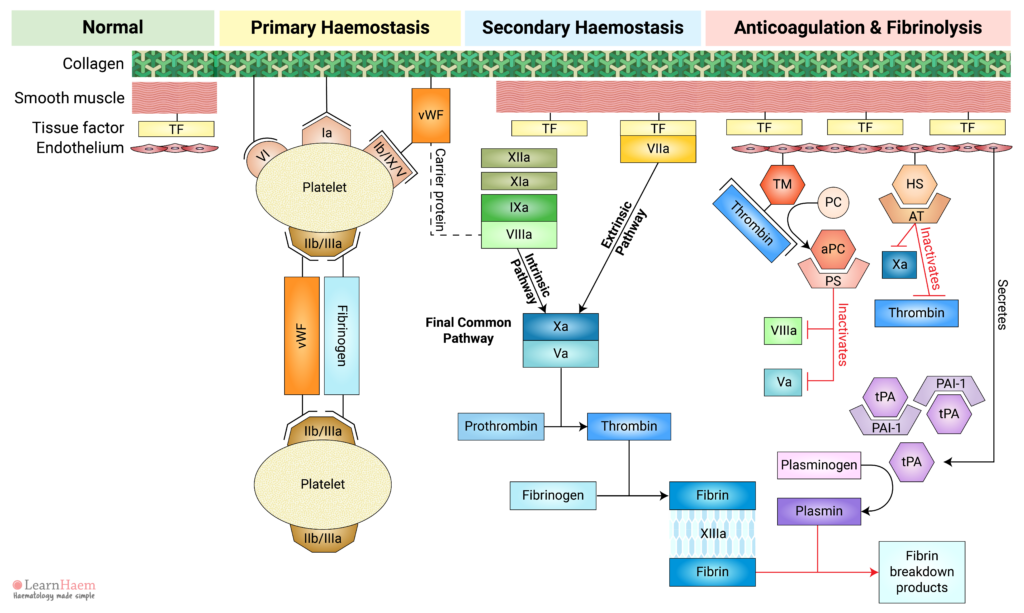
Review primary and secondary haemostasis, its regulation by natural anticoagulants and normal clot breakdown via fibrinolysis. This outlines the process by which injury results in clot formation at the site of injury and prevention of pathological clot progression in normal areas.
The process is outlined step-by-step below. You can also watch the embedded video above if you prefer.
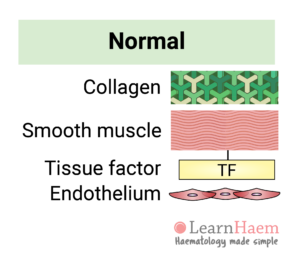
1. The Blood Vessel
The normal blood vessel comprises collagen, smooth muscle and an endothelial layer.
Smooth muscle cells express tissue factor and secrete components of the extracellular matrix, such as collagen. The endothelial layer acts as a natural barrier between blood and the tissue factor expressed by smooth muscle.
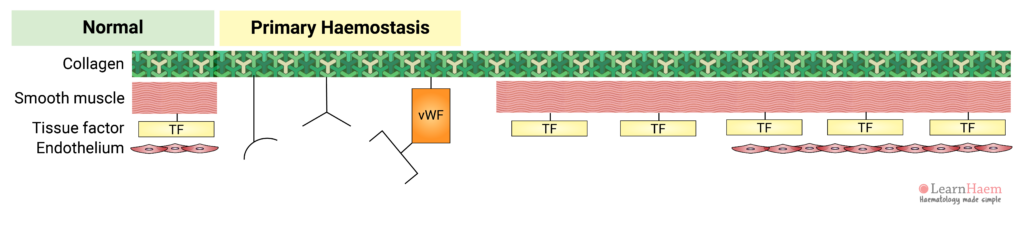
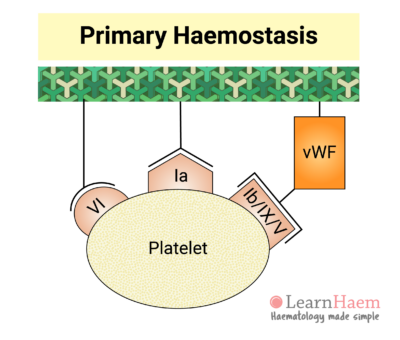
3. Platelet Adhesion
This triggers platelet adhesion via receptors which bind directly to collagen (GP VI and GP Ia) and receptors which bind to von Willebrand Factor (vWF).
Inactive vWF exists in a coiled form. Exposure of vWF to flowing blood subjects it to shear stress, causing it to uncoil and exposing platelet receptor Ib/IX/V binding sites.
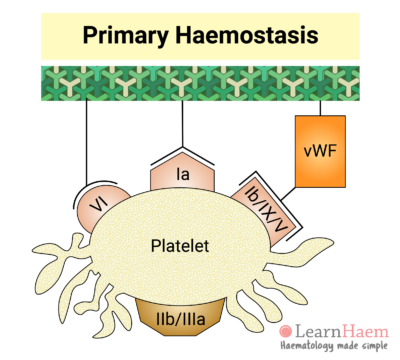
4. Platelet Activation
Binding of platelets to their sub-endothelial receptors results in platelet activation. Platelet activation triggers a reorganisation of the platelet cytoskeleton, resulting in a shape change. This increases the surface area upon which further adhesion, aggregation and coagulation can occur.
Platelet activation also results in a conformational change in the GP IIb/IIIa receptor, which increases its affinity for its primary ligand, fibrinogen.
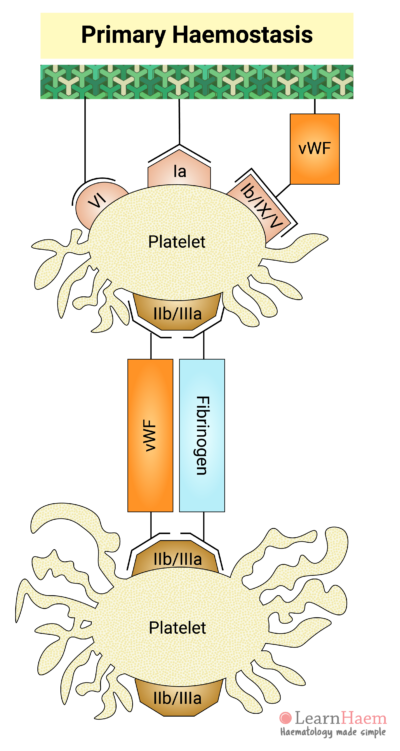
5. Platelet Aggregation
Fibrinogen acts as a bridge between platelets, facilitating the interaction of adjacent platelets, resulting in platelet aggregation.
vWF can also bind directly to GP IIb/IIIa to facilitate further platelet adhesion and aggregation at the site of injury.
The formation of a platelet plug at the site of vascular injury is known as primary haemostasis.
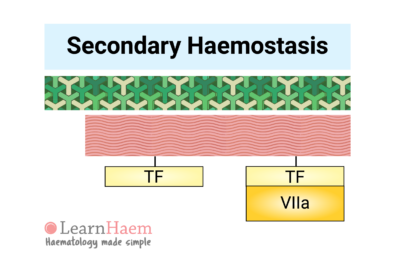
6. The Extrinsic Pathway
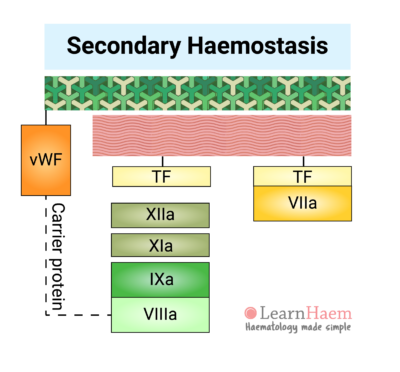
Secondary haemostasis refers to the cascade of reactions resulting in the formation of fibrin.
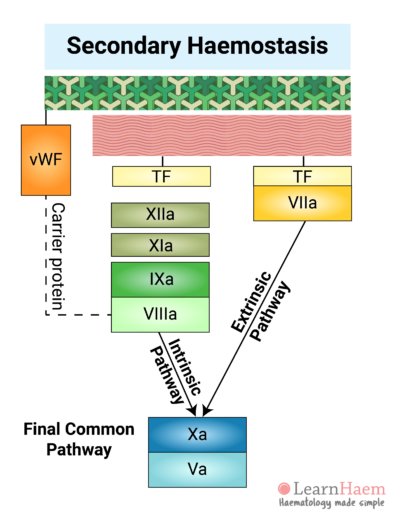
Vessel injury results in the exposure of tissue factor (TF) to factor VII circulating in the blood. Binding of factor VII to TF results in the activation of factor VII (VIIa). The TF-VIIa complex is known as the extrinsic tenase. This will be explained in the section on the cell-based model of coagulation.
This part of the cascade is known as the extrinsic pathway.
7. The Intrinsic Pathway
Factors XII, XI, IX and VIII make up the intrinsic pathway of the coagulation cascade. Contact activators result in the activation of factor XII (XIIa), which then converts factor XI into activated FXI (XIa).
XIa then activates factor IX (IXa), which combines with its co-factor activated factor VIII (VIIIa) to form the intrinsic tenase complex.
vWF is also tightly bound to factor VIII under normal circumstances, protecting it from rapid degradation.
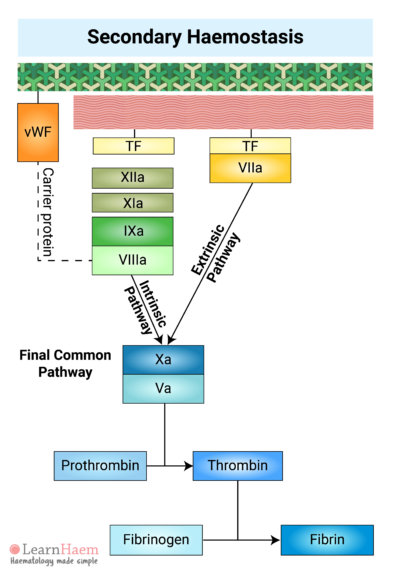
8. The Final Common Pathway
The intrinsic and extrinsic pathways converge on the final common pathway. The intrinsic and extrinsic tenases catalayse the formation of activated factor X (Xa) from its inactive form.
Xa forms a complex with its co-factor, activated factor V (Va), known as the prothrombinase complex.
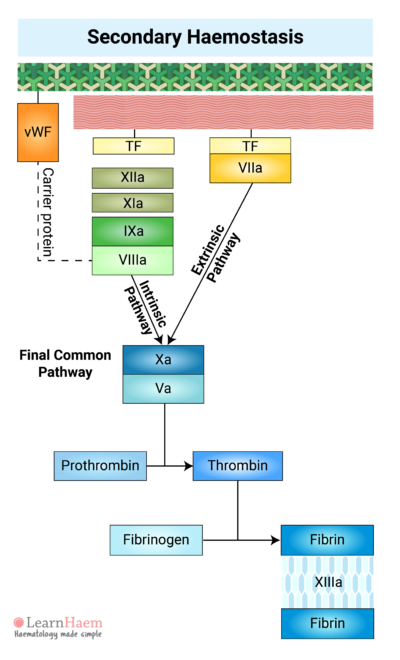
10. Factor XIII Facilitates the Formation of a Fibrin Mesh
Fibrin generated in the final common pathway of the coagulation cascade cross-links with each other to form a fibrin mesh.
Factor XIII stabilises the fibrin mesh by catalysing the cross-linking of fibrin. This reinforces the fibrin mesh against shear stress and also protects it from lysis by plasmin.
The fibrin mesh reinforces the platelet plug formed during primary haemostasis, giving it extra tensile strength.
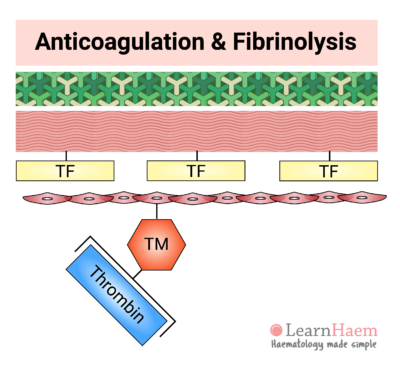
11. Termination of Coagulation: Thrombomodulin
Away from the site of injury, haemostasis must be terminated to prevent pathological clot progression (thrombosis). This is achieved through activation of the natural anticoagulants.
Normal endothelial cells express thrombomodulin (TM). When thrombin that is generated in the coagulation cascade comes into contact with thrombomodulin, it binds and results in a confirmational change in thrombomodulin. This changes the substrate specificity of thrombin from fibrinogen to protein C.
This means that thrombin exerts an anticoagulant effect when the endothelium is intact (i.e., away from sites of injury), but a pro-coagulant effect at sites of injury.
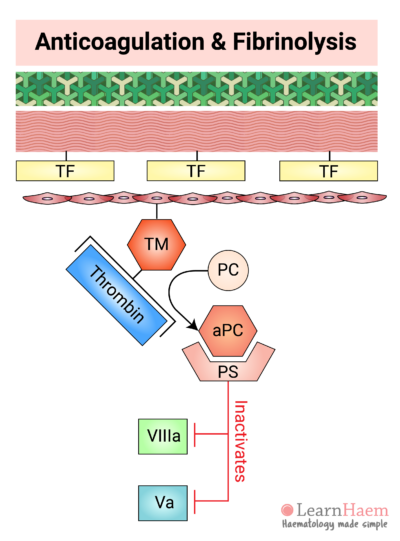
12. Activated Protein C Degrades Va and VIIIa
The thrombin-TM complex activates protein C (aPC), which binds to its co-factor, protein S (PS).
The aPC-PS complex inactivates both activated factor V and activated factor VIII, which are largely responsible for thrombin and activated factor X generation, respectively.
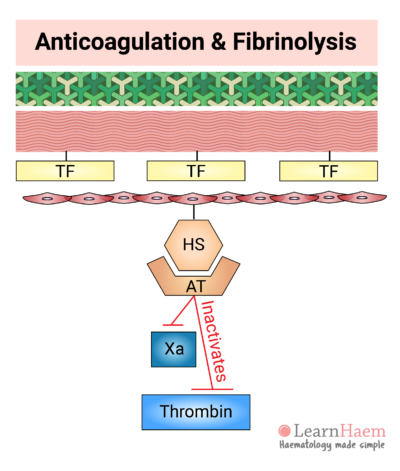
13. Antithrombin Degrades Xa and Thrombin
Normal endothelial cells also express heparan sulphate (HS). HS binds to circulating antithrombin (AT), triggering a conformational change in AT which markedly increases its affinity for activated factor X and thrombin.
Binding of AT to Xa and thrombin results in the inactivation of these factors.
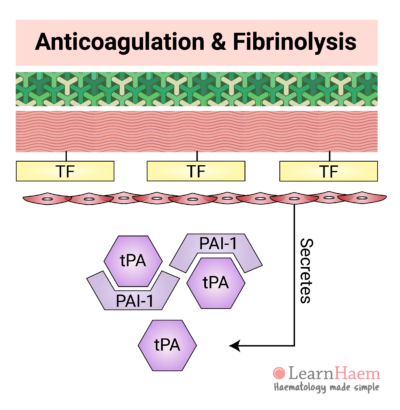
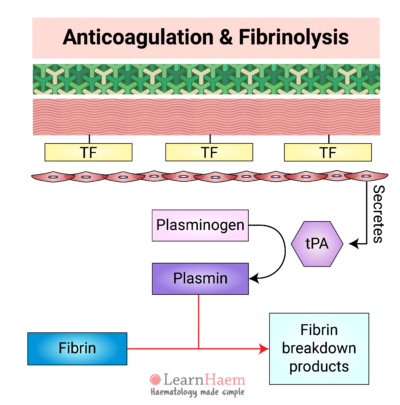
14. Tissue Plasminogen Activator
Normal endothelial cells secrete tissue plasminogen activator (tPA). Under normal circumstances, tPA is inhibited by plasminogen activator inhibitor-1 (PAI-1). However, in response to thrombin, endothelial secretion of tPA increases. This results in saturation of PAI-1 and excess tPA.
15. Plasmin Lyses Fibrin
tPA converts inactive plasminogen to plasmin. Plasmin then lyses fibrin, resulting in the formation of fibrin breakdown products such as D-dimers and serum cross-linked fibrin (XDP).
Fibrinolysis and the natural anticoagulants ensure the clot remains limited to the site of injury.

EXCELLENT MATERIAL
THANKYOU
awesome presentations..
Very nice and simple cartoon diagram