For sample collection, use venous blood where possible, with a tourniquet applied for as short a time as possible (venous occlusion increases fibrinolytic activity, activates clotting factors and causes haemoconcentration).
Sample collection timing post-factor administration is important, as they have varying peaks and troughs:
- Factor VIII: 15min
- Factor IX: 30min
Samples should be transported to the lab as quickly as possible to prevent degradation of labile factors such as factor V and VIII.
Most routine assays require platelet poor plasma (PPP): centrifuge at 2000g for 15min.
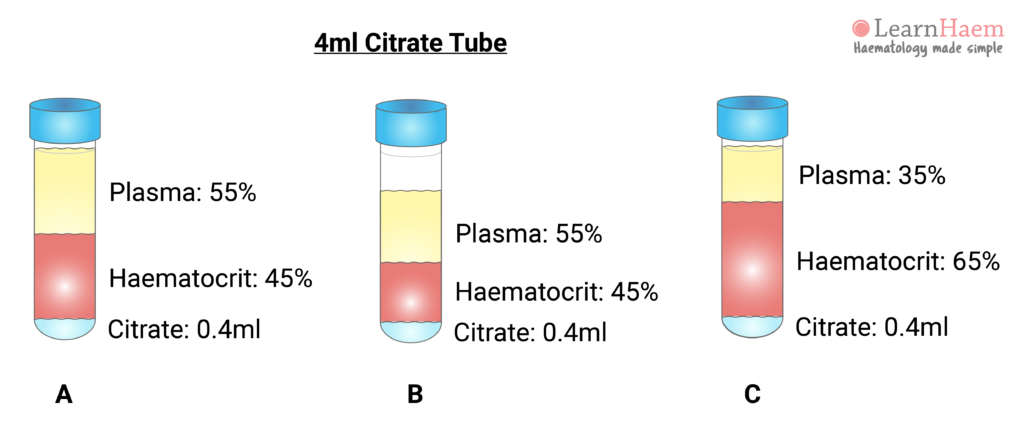
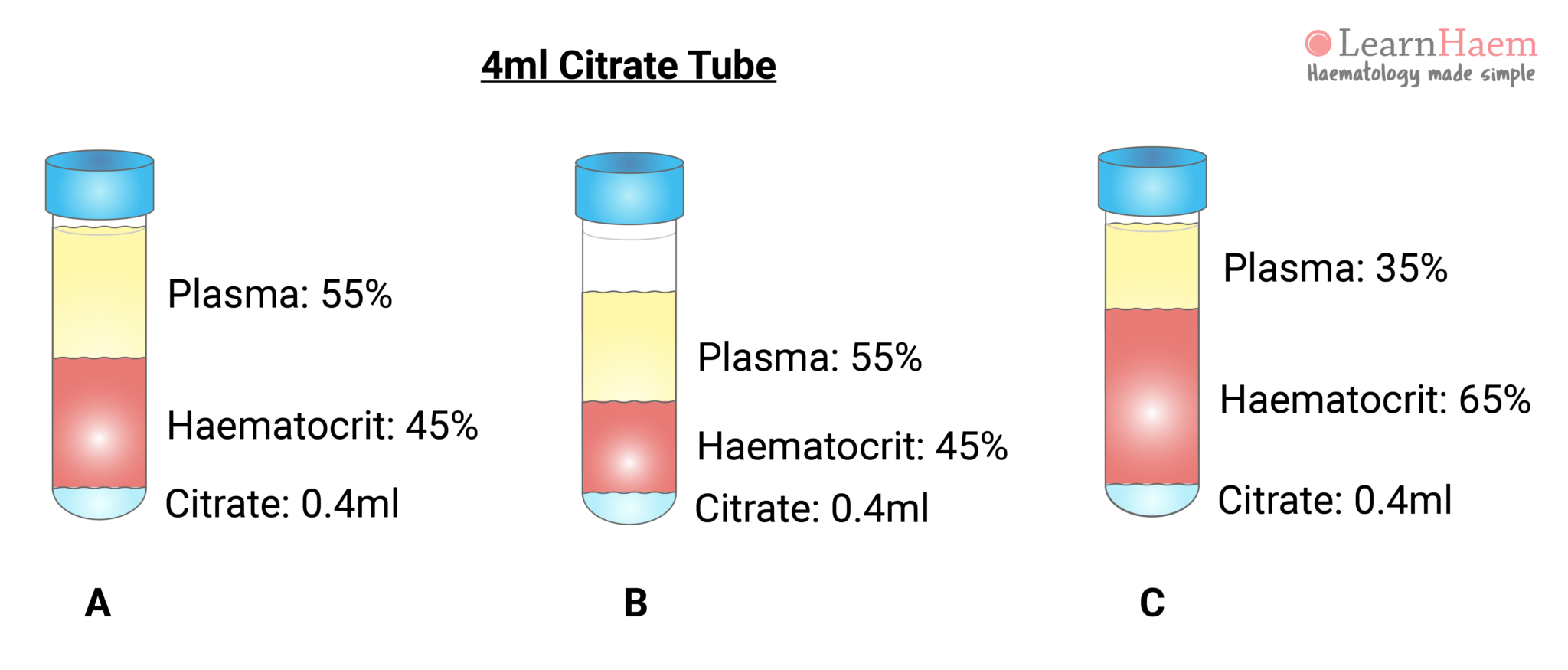
Samples for coagulation tests are usually collected in a blue Vacutainer tube. This contains a citrate anticoagulant. For routine coagulation assays, nine blood volumes are added to one volume of citrate. The ratio of citrate to plasma must be correct in order for the tests to be accurate (sample A). In sample A, representing a normal patient, the coagulation tube is filled to the mark. Normal plasma comprises ~55% of the blood volume (or about 2.2ml of the tube), giving a plasma : citrate ratio of 5.5.
Sample B illustrates how the ratio of plasma to citrate changes when the tube is incorrectly filled. If only 3ml of blood is collected, then 1.65ml is plasma. This changes the plasma : citrate ratio to 4.1. Sample C illustrates the effect of erythrocytosis on the plasma : citrate ratio, even if the tube is correctly filled. A patient with 35% plasma (or 1.4ml in a coagulation tube) will have a plasma : citrate ratio of 3.5.
This explains why coagulation tests may be inaccurate in certain pathological states, such as dehydration, polycythaemia and marked leukocytosis (the latter expands the buffy coat layer). The amount of citrate in the tube requires adjustment for the patient’s haematocrit. The effect of the plasma : citrate ratio on coagulation test results is more marked at extreme of haematocrit.

Leave A Comment